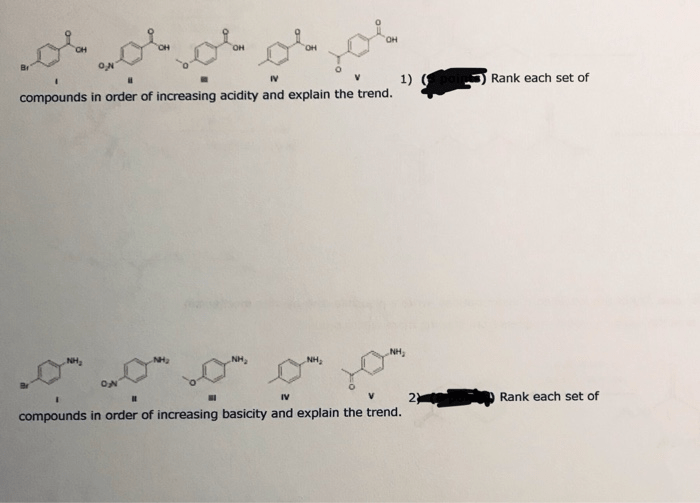
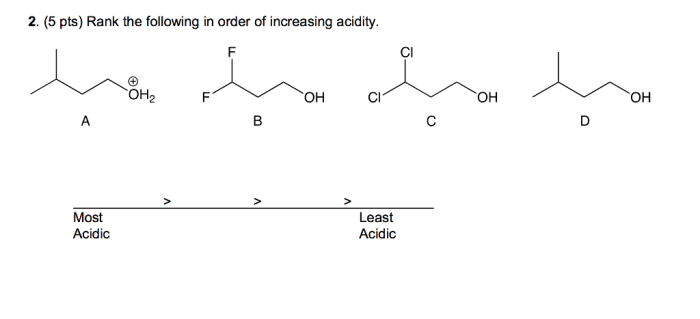
Rank each set of compounds in order of increasing acidity sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. Acidity, a fundamental concept in chemistry, plays a pivotal role in shaping the behavior and properties of compounds, and understanding its intricacies is crucial for unraveling the complexities of the chemical world.
In this comprehensive guide, we embark on a journey to explore the factors that govern acidity, unravel the methods employed to determine it, and delve into the diverse applications of acidity ranking in various scientific disciplines. Along the way, we will encounter limitations and challenges, but we will also discover strategies to overcome them, equipping ourselves with a deeper understanding of this fascinating aspect of chemistry.
Ranking Compounds by Acidity
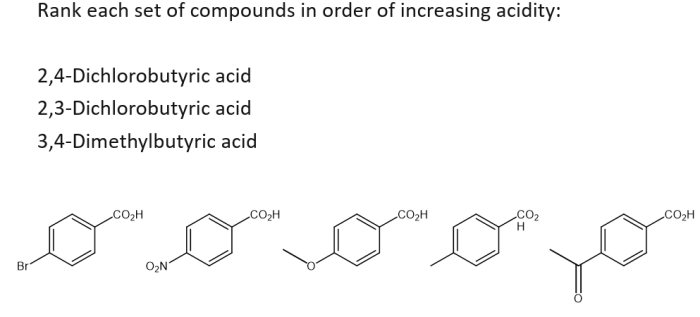
Acidity is a measure of the ability of a compound to donate protons (H+ ions). It is an important property in chemistry as it affects the reactivity of compounds and their behavior in various chemical reactions.The acidity of a compound is determined by several factors, including:
- The strength of the bond between the proton and the rest of the molecule.
- The stability of the conjugate base formed when the proton is donated.
- The solvent in which the compound is dissolved.
Procedure for Ranking Compounds by Acidity, Rank each set of compounds in order of increasing acidity
To rank a set of compounds in order of increasing acidity, follow these steps:
- Identify the conjugate base of each compound.
- Compare the stability of the conjugate bases. The more stable the conjugate base, the more acidic the compound.
- Rank the compounds in order of increasing acidity, based on the stability of their conjugate bases.
| Compound | Conjugate Base | Stability of Conjugate Base | Acidity |
|---|---|---|---|
| HCl | Cl- | Very stable | Strong acid |
| CH3COOH | CH3COO- | Less stable than Cl- | Weak acid |
| NH4+ | NH3 | Less stable than CH3COO- | Very weak acid |
Quick FAQs: Rank Each Set Of Compounds In Order Of Increasing Acidity
What factors influence the acidity of a compound?
The acidity of a compound is primarily determined by the stability of its conjugate base. Factors such as electronegativity, resonance, and inductive effects play a crucial role in stabilizing the conjugate base, thereby influencing the acidity of the compound.
How can we determine the acidity of a compound?
There are several methods to determine the acidity of a compound, including pH measurement, titration, and spectroscopic techniques. Each method offers unique advantages and limitations, and the choice of method depends on the specific compound and the desired accuracy.
What are the applications of acidity ranking?
Acidity ranking finds applications in various fields, including organic chemistry, biochemistry, and environmental science. It helps predict the reactivity of compounds, understand biochemical processes, and assess the environmental impact of acidic substances.